Effects of the Doctor-Designed Proprietary 6-Week Weight Loss Plan on Weight and Body Size Among Overweight Adults
Abstract
Objective: The purpose of this study was to report on the effects of the Doctor-Designed Proprietary 6-Week Weight Loss Plan on weight and anthropometric measurements among overweight adults. The Doctor-Designed Proprietary 6-Week Weight Loss Plan is a 42-day weight loss program that employs the Proprietary Loss Plan nutritional support formula and the Proprietary Loss Plan eating program.
Methods: This was an observational study that employed a pre-post intervention design without a control group. Practitioners known to prescribe the Doctor-Designed Proprietary 6-Week Weight Loss Plan to their patients were surveyed and asked to provide data on weight change after one week of being on the program, weight change after completing one cycle of the program (change at 6 weeks), and changes in anthropometric measurements after completing one cycle of the program. One sample t-tests were used to investigate statistically significant mean changes from 0 and one sample Wilcoxon signed-rank tests were used to investigate median changes from 0.
Results: Data on 573 individuals (349 females and 224 males) that completed the Doctor-Designed Proprietary 6-Week Weight Loss Plan was analyzed. At one-week, the mean weight reduction was 8.9 pounds (95% CI = 8.6 to 9.2) and the median reduction was 8.6 pounds (95% CI = 8.4 to 9.0). At six-weeks, the mean weight reduction was 25.1 pounds (95% CI = 24.4 to 25.8) and the median reduction was 24.4 pounds (95% CI = 23.8 to 25.0). Further, the mean reduction in body size (sum of all anthropometric measurements) was 19.5 (95% CI = 18.9 to 20.0) inches and the median reduction was 19.0 (95% CI = 18.4 to 19.6) inches. All mean and median results were statistically significant (P < 0.001).
Conclusion: The results indicate that the Doctor-Designed Proprietary 6-Week Weight Loss Plan is an effective treatment for individuals struggling with excess weight. Positive results of the Doctor-Designed Proprietary 6-Week Weight Loss Plan were observed within one week of initiating the program and continued through the full six weeks.
Introduction
The proportion of adults in the United States defined as overweight or obese (BMI ≥ 25) has risen from around 50% in the early 1980’s to over 70% today.1 A likely reason for this increase is the continued societal progression towards excess energy intake and inactivity.2,3 As a result, obesity and obesity-related medical problems, such as heart disease, stroke, and type 2 diabetes, are an issue across nearly every group in the United States.4 Thus, the majority of adult Americans are in need of an effective anti-obesity treatment.
Accumulating evidence indicates that calorie-restricting diets that are implemented by addressing portion size and food nutritional quality may be an effective treatment for obesity.2 For example, studies have shown that these types of diets may allow individuals to more effectively lose weight, maintain a healthy weight, and prevent chronic diseases associated with obesity.5-8 Ultimately, however, adherence is the critical factor that will determined the effectiveness of any anti-obesity treatment.3,9 Thus, patient education is a key element to incorporate into an anti-obesity treatment strategy.10-12 One option that aims to treat obesity by implementing each of these aspects (low-calorie, portion size, dietary energy density, and patient education) into an overall treatment program is the Doctor-Designed Proprietary 6-Week Weight Loss Plan.
The Doctor-Designed Proprietary 6-Week Weight Loss Plan consists of an eating program that utilizes specific amounts, blends, and ratios of protein and complex carbohydrate food sources along with the implementation of daily intermittent fasting. In this way, the program aims to incorporate a strategy that combines calorie restriction with portion size control and an improvement in diet quality as it relates to energy density.2,5 Additionally, the Proprietary Loss Plan eating program teaches participants how to make food choices that aid in the promotion of long-term weight reduction by educating participants on behavioral habits related to food purchases, meal preparation, and food consumption. Additionally, participants are shown how to shop for, prepare, and consume foods that promote long-term weight loss and improved health.
The Doctor-Designed Proprietary 6-Week Weight Loss Plan also implements a nutritional support formula. The Proprietary Loss Plan Nutritional Support formula is a proprietary formula that is composed of various amino acids, cell salts, and vitamins at multiple homeopathic potencies. Each ingredient has been included for its stand-alone effects at each individual potency as well as its interactions when combined with the other ingredients at their individual potencies. Further, the nutritional support formula uses only all-natural ingredients and is both stimulant-free and hormone-free. The nutritional support formula is formulated to include ingredients that have been shown to aid metabolic function, stabilize blood sugars, protect muscles, and promote detoxification.13,14 Further, the ingredients contained within the nutritional support formula aid in hormone production and regulation, thereby allowing for interactions with the Hypothalamic-Pituitary-Adrenal-Thyroid Axis to aid in the prevention of HPAT axis dysregulation.15 HPAT axis dysregulation may be the causal link between conditions such as maternal malnutrition, sleep deprivation, metabolic disease, and metabolic syndrome.13,14 As such, preventing dysregulation of the HPAT axis may help prevent obesity, especially upper body obesity and other metabolic diseases.15
The aim of this study was to assess the effects of one cycle (equal to 42 days) of the Doctor-Designed Proprietary 6-Week Weight Loss Plan on body weight and body size. Study outcomes included weight change one week after initiating the Doctor-Designed Proprietary 6-Week Weight Loss Plan, weight change at the end of the cycle (42 days after beginning the cycle) and change in body size at the end of the cycle.
Methods
Study Design and Population
This was an observational study that utilized a pre-post intervention without a control group or blinding. To be eligible for this study participants were required to be 18 years of age or older, have a BMI greater than or equal to 25, not be pregnant or breast feeding, not have cancer or be receiving treatment for cancer, and to not have had active cholecystitis within the last 4 weeks. The Doctor-Designed Proprietary 6-Week Weight Loss Plan, which served as the intervention, is offered and administered exclusively through chiropractic offices. All chiropractic offices that offer the Doctor-Designed Proprietary 6-Week Weight Loss Plan were emailed asking for their participation. Each office was asked to have their medical/chiropractic assistant(s) randomly select up to 25 male and female patients that had completed a 42-day cycle of the program. The following information was requested for each patient; gender, age, ethnicity, weight change at 1-week, weight change at 6-weeks, and change in body size at 6-weeks. Body size was assessed by taking measurements from the neck, shoulders, chest, abdomen, hips, right bicep, right thigh, and right calf.
Intervention
The intervention consists of a 42-day cycle that incorporates an intermittent/micro-fasting component, meal guidelines to follow, a nutritional support formula, and weekly visits to discuss the program. The micro-fasting component is implemented via the removal of the morning meal. Thus, participants only consume an afternoon meal and evening meal during the 42-day cycle. Meals are to meet the following guidelines: 1) both proteins and carbohydrates are required at each meal (a list of acceptable proteins and complex carbohydrates, as well as the quantity of each, is provided), 2) specific “free” vegetables (a list is provided) can be consumed in near unlimited quantity at any meal, 3) ingredients that contain fat or sugar are to be avoided while zero-calorie seasonings and sauces are acceptable, and 4) 80-120 ounces of water should be consumed per day. A recipe book is provided that contains approved meals that participants can choose from during the program or they can develop meals on their own that align with the guidelines. Participants are provided with a “Food Tracking Journal” to document their meals.
The Proprietary Loss Plan nutritional support formula is a sublingually delivered nutritional supplement that is consumed 3 times per day for 39 consecutive days (days 1-39 of the program). Participants do not consume the nutritional supplement on days 40, 41, and 42. The recommended spacing for consumption of the nutritional support formula is 7 hours apart and the recommended times for consumption are 7am, 2pm, and 9pm. Being as the nutritional support formula is delivered sublingually, participants are instructed to abstain from consuming liquids or foods 5-10 minutes before and 5-10 minutes after the formula’s administration. The six weekly follow-up visits that occur over the course of the program each include: 1) a review of the “Food Tracking Journal” with discussion related to the choices the participant made during the previous week, 2) a counselling session related to food choices, food-related behavior, what went well, what did not go well, and what can be done to improve, 3) a BMI calculation, 4) an 8-point body measurement update, 5) a blood pressure reading, and 6) a weight measurement.
Once the participant has completed the 42-day cycle, they enter a re-feeding/weight maintenance phase where they are transitioned to a more traditional food consumption schedule consisting of 3 meals per day. The participant is provided with easy-to-implement guidelines delineating the types and quantities of foods that they should continue to consume to maximize the likelihood of maintaining their weight loss. Participants are also encouraged to implement a physical activity/exercise program of their choice during this phase.
Statistical Analysis
The study population was characterized in terms of gender, age, and ethnicity. Specifically, frequencies and percentages were calculated for categorical variables (gender and ethnicity) and means and standard deviations were calculated for continuous variables (age). The relationships between each of the outcome variables and gender, age, and ethnicity were then investigated. Box plots were developed to help visualize these relationships.
The outcome variables were then investigated for statistically significant changes from zero. One-sample t-tests were applied to each outcome variable for global changes from zero and also within each gender. Additionally, as each of the outcome variables were found to have elongated tails in the negative direction (Supplemental Figure 1), the robustness of the t-test results were assessed by also conducting one-sample Wilcoxon signed rank tests. The one-sample Wilcoxon signed rank test is the nonparametric equivalent of the one sample t-test.
Results
Information was obtained on 574 participants (provided by 16 offices located in various geographical regions in the United States) that met the inclusion criteria. One participant was excluded due to a clear error in their data (weight lost at six weeks was reported as 255.2 pounds). Thus, 573 individuals were included in the analysis (349 females and 224 males). Table 1 outlines the full characteristics of the study population. The mean age was 51 years old (standard deviation = 13.2) and 84.1% of participants were white. Boxplots of each outcome variable by gender, age, and ethnicity showed fairly consistent trends across each group investigated (Figure 1). However, males generally lost more weight than females and blacks generally lost more weight than other groups.
Statistically significant (p < 0.0001) losses in weight and body size were found at each time point and for each group investigated, with both one sample t-tests and Wilcoxon signed-rank tests (Table 2). Specifically, at one-week, the mean weight reduction was 8.9 pounds (95% CI = 8.6 to 9.2) and the median reduction was 8.6 pounds (95% CI = 8.4 to 9.0). At six-weeks, the mean weight reduction was 25.1 pounds (95% CI = 24.4 to 25.8) and the median reduction was 24.4 pounds (95% CI = 23.8 to 25.0). Further, the mean reduction in body size (sum of all anthropometric measurements) was 19.5 (95% CI = 18.9 to 20.0) inches and the median reduction was 19.0 (95% CI = 18.4 to 19.6) inches.
| Characteristic |
Count (%) (n = 573) |
|---|---|
| Age, mean (std) | 51 (13.2) |
| Age groups | |
| ≤ 20 | 6 (1.0) |
| 21-35 | 69 (12.0) |
| 36-50 | 181 (31.6) |
| 51-65 | 241 (42.1) |
| 66-80 | 71 (12.4) |
| > 80 | 5 (0.9) |
| Race/ethnicity | |
| White | 482 (84.1) |
| Black | 10 (1.7) |
| Hispanic | 20 (3.5) |
| Other | 9 (1.6) |
| Missing | 52 (9.1) |
| Male | 224 (39.1) |
Table 1. Background characteristics of the study population.
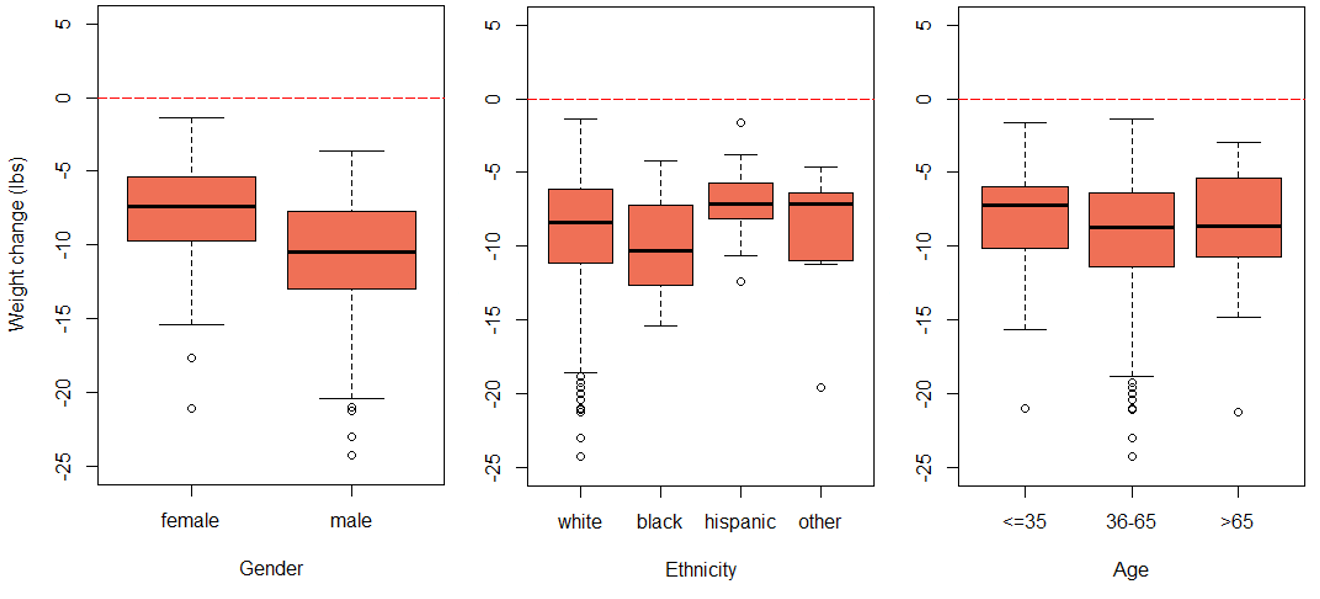
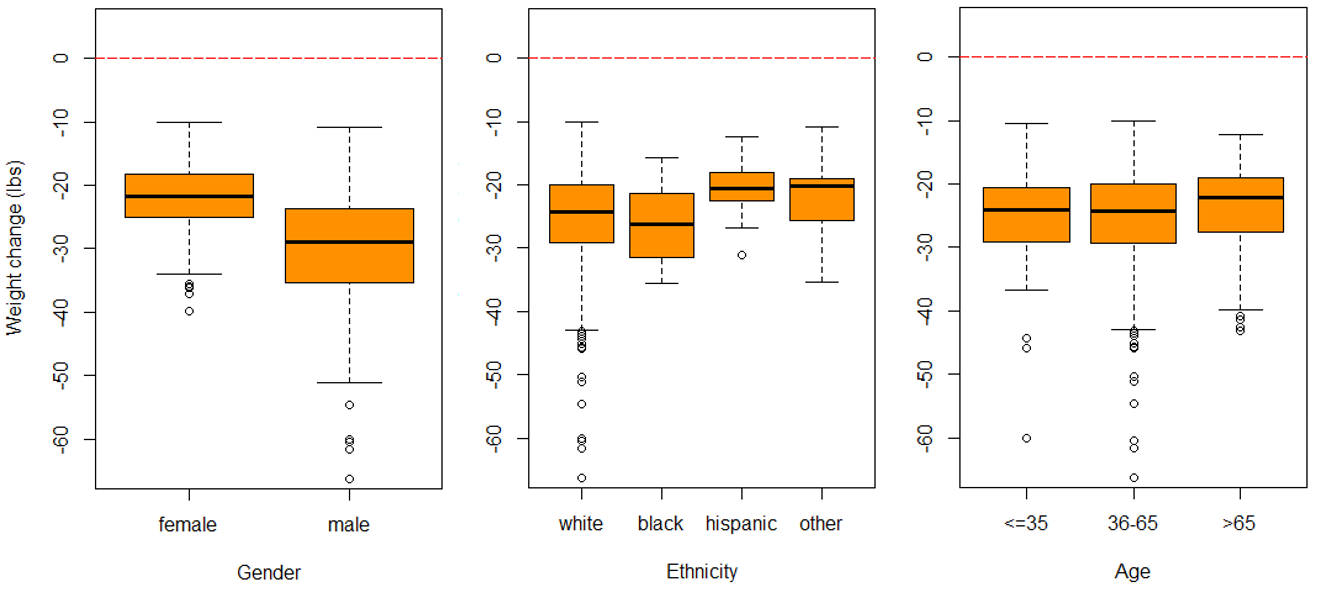
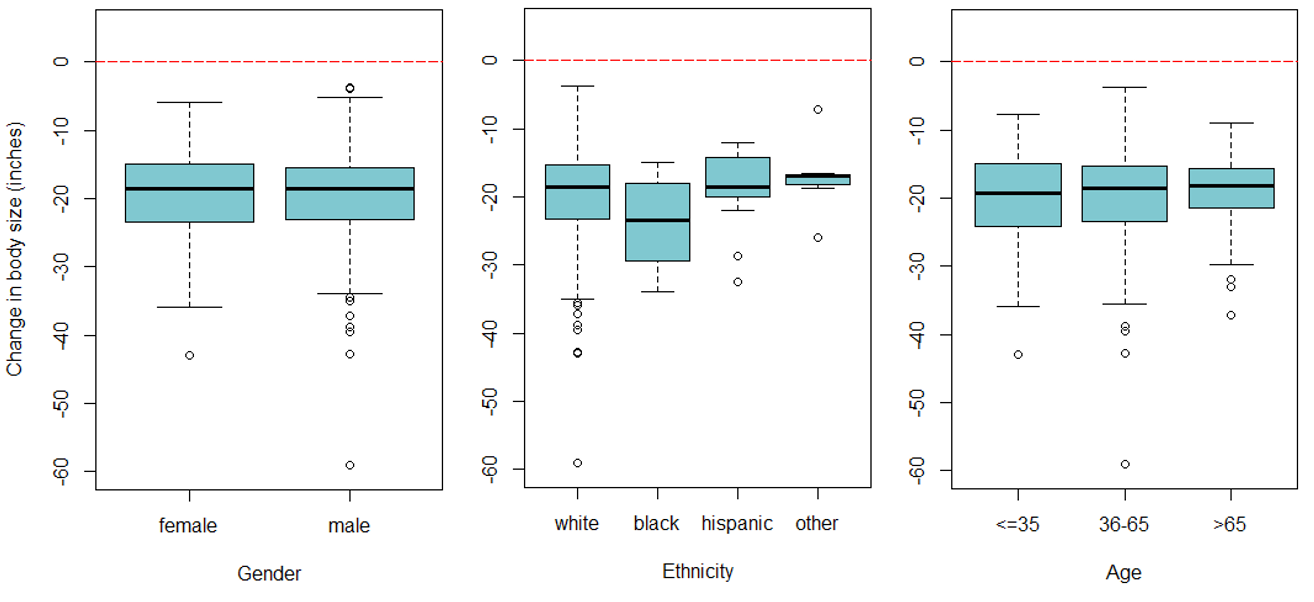
Figure 1. Boxplots of gender, ethnicity, and age on each outcome. The top panel is weight change at 1 week, the middle panel is weight change at 6 weeks, and the bottom panel is change in size at 6 weeks.
| Outcome |
T-test (mean & 95% CI) |
Wilcoxon signed-rank (median & 95% CI) |
|---|---|---|
| Weight change (lbs) 1 week (n = 573) | -8.9 (-9.2 to -8.6) | -8.6 (-9.0 to -8.4) |
| — Female (n = 349) | -7.7 (-8.0 to -7.4) | -7.5 (-7.9 to -7.2) |
| — Male (n = 224) | -10.8 (-11.3 to -10.3) | -10.5 (-11.1 to -10.0) |
| Weight change (lbs) 6 weeks (n = 573) | -25.1 (-25.8 to -24.4) | -24.4 (-25.0 to -23.8) |
| — Female (n = 349) | -22.1 (-22.6 to -21.6) | -22.0 (-22.5 to -21.4) |
| — Male (n = 224) | -29.8 (-31.0 to -28.6) | -29.3 (-30.5 to -28.1) |
| Body size change (in.) 6 weeks (n = 544) | -19.5 (-20.0 to -18.9) | -19.0 (-19.6 to -18.4) |
| — Female (n = 326) | -19.3 (-20.0 to -18.6) | -18.9 (-19.6 to -18.2) |
| — Male (n = 218) | -19.7 (-20.7 to -18.7) | -19.1 (-20.0 to -18.3) |
Table 2. Results of statistical tests to assess outcomes globally and by gender.
Discussion
This study aimed to assess the effects of one cycle (equal to 42 days) of the Doctor-Designed Proprietary 6-Week Weight Loss Plan on body weight and body size. The results indicate that the Doctor-Designed Proprietary 6-Week Weight Loss Plan is an effective treatment for individuals struggling with excess weight. Positive results of the Doctor-Designed Proprietary 6-Week Weight Loss Plan were observed within one week of initiating the program (mean weight loss of 8.9 pounds) and benefits continued through the full 6 weeks (mean weight loss of 25.1 pounds). These benefits are likely to decrease the risk of metabolic syndrome and certain diseases, including, but not limited to, cardiovascular disease, stroke, and type II diabetes.16
Recommendations suggest that overweight and obese patients should set a goal of a 10% reduction in total body weight and be encouraged to lose approximately 2% of initial body weight per week to reach the 10% goal.17-21 Thus, for an individual with a starting weight of 200 - 250 pounds (the typical average weight across men and women that use the Doctor-Designed Proprietary 6-Week Weight Loss Plan), the Proprietary Loss Plan program appears to fit perfectly with these recommendations. For example, a 2% weekly reduction in an individual with a starting weight of 225 pounds translates into 4.5 pounds per week (participants lost an average of 4.2 pounds per week in this study). Further, a 10% initial weight loss goal translates into 22.5 pounds (participants lost an average of 25.1 pounds per week in this study).
Results from other commercial programs can be compared with the findings of this study. A study investigating the Weight Watchers program reported a mean weight loss of 5.1% of initial body weight at 26 weeks.22 In a separate study that investigated the eDiets.com program, the eDiet intervention lost 0.9% of initial body weight at 16 weeks.23 A study that investigated the Jenny Craig program reported a mean weight loss of 7.8% of initial body weight at 26 weeks.24 Lastly, a study that investigated the Beachbody weight loss program reported a weight loss of 8.7 pounds over a 21-day period.5 While the study populations and endpoints employed in these other studies differ from the current study, the Proprietary Loss Plan program investigated here appears to outperform other commercial weight loss programs.
Limitations
This study had several limitations. First, the lack of blinding, randomization, and a control group limits the generalizability of the results. Second, even though Practitioners were encouraged to randomly select which eligible patients to provide data for, potential participant selection bias by the Practitioners that provided the data could further limit the generalizability of the results. Third, the lack of baseline weight and body size measurements precluded an investigation into the effectiveness of the program as a function of initial weight (or percentage of weight lost). However, our results do show that the program was generally effective for each gender, age, and ethnicity examined. Lastly, this study only investigated the effects on patients that successfully completed a 42-day cycle. Thus, the overall effectiveness among all eligible patients that initially enrolled in the program is likely lower. However, participants generally tolerate the program well and as result program adherence is likely high.
Conclusion
The findings from this exploratory study suggest that the Doctor-Designed Proprietary 6-Week Weight Loss Plan may facilitate weight loss and a reduction in body size. Future studies should address both the longer-term effects of a single cycle of the program and also the potential additive effects of completing multiple cycles. Further, studies on participant adherence would be informative.
Funding sources and potential conflicts of interest
Author developed the Doctor-Designed Proprietary 6-Week Weight Loss Plan.
References
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311(8):806–14.
- Rolls BJ. Plenary lecture 1: dietary strategies for the prevention and treatment of obesity. Proc Nutr Soc 2010;69:70–9.
- Malik VS, Hu FB. Popular weight-loss diets: from evidence to practice. Nat Clin Pract Cardiovasc Med 2007;4:34–41.
- Ahima RS, Lazar MA. Physiology: the health risk of obesity—better metrics imperative. Science 2013;341(6148):856-858.
- Balliett DC, Burke JR. Changes in anthropometric measurements, body composition, blood pressure, lipid profile, and testosterone in patients participating in a low-energy dietary intervention. Journal of Chiropractic Medicine 2013;12:3–14.
- Astrup A, Dyerberg J, Selleck M, Stender S. Nutrition transition and its relationship to the development of obesity and related chronic diseases. Obes Rev 2008;9(Suppl 1):48–52.
- Raynor HA, Van Walleghen EL, Bachman JL, Looney SM, Phelan S, Wing RR. Dietary energy density and successful weight loss maintenance. Eat Behav 2011;12:119–25.
- Rolls BJ, Roe LS, Beach AM, Kris-Etherton PM. Provision of foods differing in energy density affects long-term weight loss. Obes Res 2005;13:1052–60.
- Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005;293:43–53.
- Glanz K, Hersey J, Cates S, Muth M, Creel D, Nicholls J, et al. Effect of a nutrient rich foods consumer education program: results from the nutrition advice study. J Acad Nutr Diet 2012;112:56–63.
- Masheb RM, Grilo CM, Rolls BJ. A randomized controlled trial for obesity and binge eating disorder: low–energy-density dietary counseling and cognitive-behavioral therapy. Behav Res Ther 2011;49:821–9.
- Piscopo S. The Mediterranean diet as a nutrition education, health promotion and disease prevention tool. Public Health Nutr 2009;12:1648–55.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–81.
- Nemeroff CB. Clinical significance of psychoneuroendocrinology in psychiatry: focus on the thyroid and adrenal. J Clin Psychiatry. 1989;50:13–20. discussion 21–22.
- Sabio G, Davis RJ. cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem. Sci. 2010;35:490–496. doi: 10.1016/j.tibs.2010.04.004
- Garber AJ. The metabolic syndrome. Med Clin North Am. 2004;88:837–46.
- Very low-calorie diets. National Task Force on the Prevention and Treatment of Obesity, National Institutes of Health. JAMA. 1993;270:967–74.
- Furlow EA, Anderson JW. A systematic review of targeted outcomes associated with a medically supervised commercial weight-loss program. J Am Diet Assoc. 2009;109:1417–21.
- Munro IA, Garg ML. Weight loss and metabolic profiles in obese individuals using two different approaches. Food Funct. 2011;2:611–6.
- Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring) 2006;14: 1283–93.
- Orzano AJ, Scott JG. Diagnosis and treatment of obesity in adults: an applied evidence-based review. J Am Board Fam Pract. 2004;17:359–69.
- Heshka S, Greenway F, Anderson JW, Atkinson RL, Hill JO, Phinney SD, Miller-Kovach K, Xavier Pi-Sunyer F. Self-help weight loss versus a structured commercial program after 26 weeks: A randomized controlled study. Am J Med. 2000;109:282-287.
- Womble LG, Wadden TA, McGuckin BG, Sargent SL, Rothman RA, Krauthamer-Ewing ES. A randomized controlled trial of a commercial internet weight loss program. Obes Res. 2004;12:1011-1018.
- Rock CL, Pakiz B, Flatt SW, Quintana EL. Randomized trial of a multifaceted commercial weight loss program. Obesity. 2007;15:939-949
Supplemental
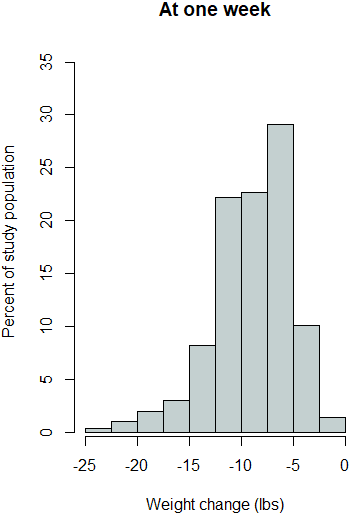
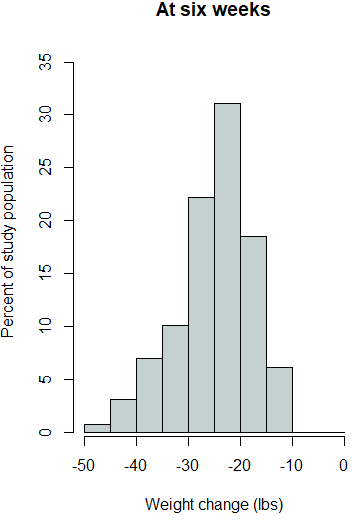
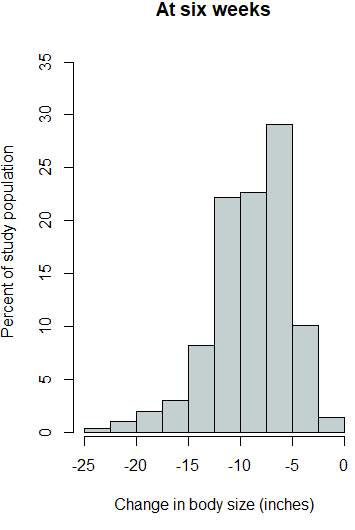
Supplemental Figure 1. Histograms for each outcome variable.
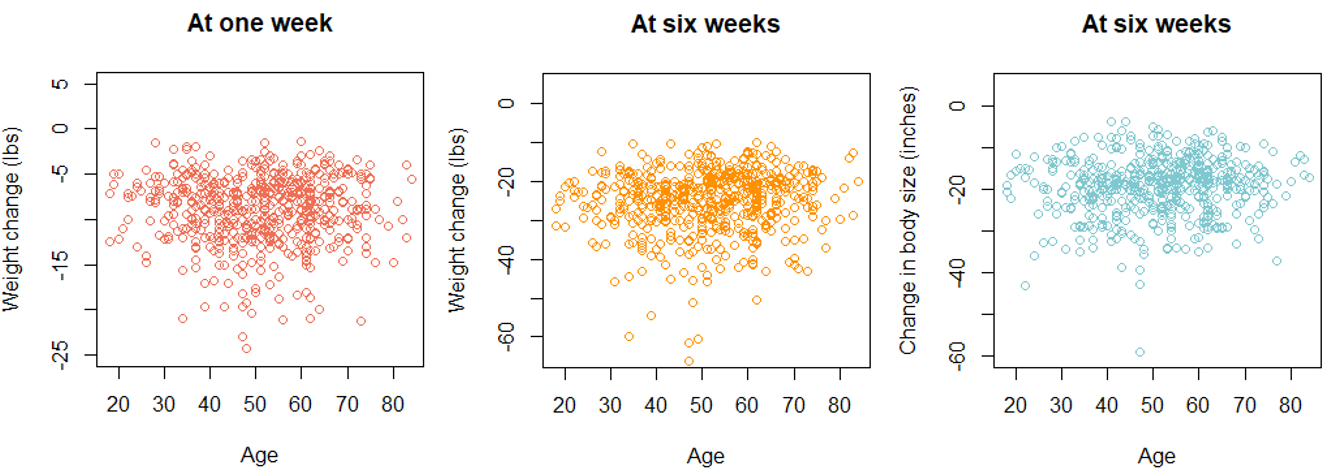
Supplemental Figure 2. Scatter plots for each outcome variable by age.
